
- Dr Vanessa Newman
Dementia is the leading cause of disability in people over 60 years old. Imaging is increasingly used to diagnose dementia to complement physical, cognitive and mental examinations.
Here, Dr Vanessa Newman explores the role of imaging in detecting this cruel and debilitating illness that effects over one million people in the UK.
Dementia: a global burden
Dementia is a leading cause of disability in people aged >60 years, representing a significant burden on patients in terms of quality of life, disability and mortality associated with the condition. This further impacts caregivers, health services and society in general. According to the World Alzheimer Report 2015, it is estimated there are 46.8 million people living with dementia worldwide and this number is due to double every 20 years. Of the 9.2 million people with dementia in Europe over 1.03 million live in the UK, representing a considerable health economic burden. Furthermore, general improved life expectancy of the global population is anticipated to correspond with increased prevalence of dementia.[1,2]
The impact of dementia on informal caregivers – such as family members and friends – is substantial and can result in physical and mental illness, social isolation and poor quality of life for them. Although their participation in the care of dementia patients may alleviate burden on healthcare systems and residential care homes, informal caregiving is not without societal costs caused by absenteeism from work.[2]
Different forms of dementia
Dementia is a progressive illness that affects not only a person’s memory but also their behaviour, mood, cognition and ability to perform daily activities. Progression of dementia is associated with both genetic predisposition and lifestyle factors, including smoking, alcohol, exercise and diet. There are a number of different dementia subtypes with varying incidence in the population, including vascular dementia (VaD), dementia with Lewy bodies (DLB), frontotemporal dementia (FTD), Parkinson’s dementia (PD) and mixed dementia. However, Alzheimer’s disease (AD) is the most prevalent form, representing 62% of the dementia population.[3–6]
Diagnosing dementia
Although the majority of patients are diagnosed with dementia in later life, evidence shows that irreversible, pathological changes within the brain occur long before the onset of clinical symptoms. Gradual changes within the brain lead to progressive cognitive impairment and patients often experience a transitional period of mild cognitive impairment (MCI), during which a differential diagnosis may not be possible.[3,7–10]
Formal assessment of cognitive decline, as undertaken by dementia experts, usually includes physical, cognitive and mental examinations [e.g. the Mini Mental State Examination (MMSE)], plus a review of education and functional levels, medications and health history.[4,11]
Dementia assessment using brain biomarkers and structural imaging
There are several protein deposition biomarkers that may be used to assist in a diagnosis of dementing diseases, such as the presence of TDP-43 (FTD), Lewy bodies (DLB), alpha-synuclein (Parkinson’s disease), plus tau and β-amyloid which are typical in the pathogenesis of Alzheimer’s disease (although not exclusive to this dementia subtype).[12,13] Historically, reliable diagnoses might only be made post-mortem using histopathology. However, increasingly the imaging of biomarkers or their effect on the living brain can be made earlier on in the course of disease, before evidence of memory impairment is seen.[12,13]

Fig 1. Source: Jovalekic et al. EJNMMI Radiopharmacy and Chemistry (2017) 1:11. doi:10.1186/s41181-016-0015-3 (Copyright held by Piramal Lifesciences).
Cerebrospinal fluid (CSF) sampling via lumbar puncture can help detect abnormal levels of soluble β‑amyloid42, total tau (T-tau) and phosphorylated tau (p-tau181), which may assist during the diagnostic workup of dementia patients being assessed for AD.[14] However, lumbar puncture is an invasive method and some patients may refuse the procedure or are contraindicated, for example, if they receive anticoagulant medications. In addition, CSF-based analyses show variability between immunoassay platforms and biomarker concentrations, which may present challenges to clinicians.[14–17]
Brain imaging in patients can assist a clinical diagnosis by examining presence of cerebral pathologies and structural changes, including MRI and CT that can detect subcortical vascular changes. Single-photon emission CT (SPECT) measuring perfusion can help differentiate AD, VaD and FTD,[4,11] while 2-(18F)Fluoro-2-deoxy-d-glucose positron emission tomography (FDG PET) may assist in detecting impaired neuronal activity by measuring the cerebral metabolic rate of glucose. This has been used to detect abnormal patterns in the brain and the potential to predict conversion from MCI to AD or the diagnosis of AD has been demonstrated.[8,9,18–20] Both SPECT-perfusion imaging and FDG-PET are indirect measures of disease that detect characteristic changes in glucose and oxygen metabolism. However, these imaging modalities show limitations in reflecting the aetiology of prodromal or mild AD.[8,9,11,19,20]
Brain β-amyloid (Aβ) deposition and plaque formation occurs early in the pathogenesis of AD, therefore offering the potential to assist in an early clinical diagnosis of patients being evaluated for Alzheimer’s dementia and other forms of cognitive impairment. Amyloid-PET is a relatively recent imaging modality and three 18F-labelled imaging agents are licensed for use in the EU that can detect the presence of β-amyloid neuritic plaques in the living brain, with validated visual assessment methods using histopathology as the standard of truth (Fig.2).[13,21] According to published appropriate use criteria, amyloid-PET is considered to have greatest utility in a subset of dementia patients:[22–24]
- where there is an established persistent or progressive unexplained memory impairment (unclear diagnosis); or
- where brain Aβ is a diagnostic consideration based on core clinical criteria, and where knowledge of this pathology may alter patient management; or
- with progressive dementia and atypical age of onset (usually <65 years of age).
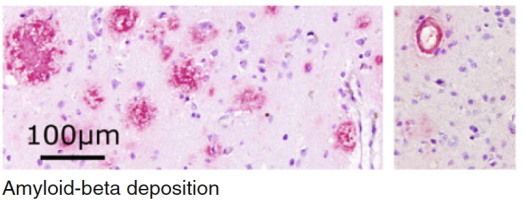
Fig 2: 18F-labelled imaging agents have the ability to detect the presence of β-amyloid neuritic plaques in the living brain (immunohistochemistry with monoclonal 6E10 Aβ antibody).[13]
Fig. 2: Source: Jovalekic et al. EJNMMI Radiopharmacy and Chemistry (2017) 1:11. doi:10.1186/s41181-016-0015-3 (Copyright held by Piramal Lifesciences).
Amyloid-PET does not alone provide a diagnosis, rather it forms part of the greater assessment workup by clinical experts, including neurologists, psychiatrists and geriatricians. The knowledge of the presence or absence of β-amyloid plaques has been shown to support a confident differential diagnosis and a tailored patient care plan, including use of medications where appropriate. There is also added value for patients and their caregivers in knowing the cause of dementia, enabling decision-making and planning for the future including the possibility of enrolling into clinical trials.[5,6,8,22–28]
The future of diagnostic imaging
The National Institute for Health and Care Excellence (NICE) is reviewing guidance on the organisation and delivery of diagnostic services, due for publication in August 2017. The scope of the revised guidance will encompass imaging in neurodegenerative diseases, as part of the wider radiology/nuclear medicine service in the NHS. This will affect not only patients, but all staff who use, refer and interpret diagnostic services in both primary, secondary and tertiary care.[29]
Author: Vanessa Newman (MD-V, PhD), Medical Affairs Director at Piramal Imaging Ltd
References
- Alzheimer-Europe, The prevalence of dementia in Europe. 2015, Alzheimer Europe: Luxembourg.
- Prince, M., World Alzheimer Report 2015: The Global Impact of Dementia – an analysis of prevalence, incidence, cost and trends, A.s.D.I. (ADI), Editor. 1015: London.
- Prince, M., World Alzheimer Report 2014: Dementia and Risk Reduction – an analysis of protective and modifyable factors, A.s.D. International, Editor. 2014, Alzheimer’s Disease International (ADI): London, UK.
- NICE, Clinical guideline 42: Dementia: Supporting people with dementia and their carers in health and social care. 2006, National Institute for Health and Care Excellence (NICE): London, UK.
- Deckers, K., et al., Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observational studies. Int J Geriatr Psychiatry, 2015. 30(3): p. 234-46.
- Kivipelto, M. and F. Mangialasche, Alzheimer disease: To what extent can Alzheimer disease be prevented? Nat Rev Neurol, 2014. 10(10): p. 552-3.
- Catafau, A.M. and Bullich, S., Amyloid PET imaging: applications beyond Alzheimer’s disease. Clin Transl Imaging, 2015. 3(1): p. 39-55.
- Sabri, O., et al., Florbetaben PET imaging to detect amyloid beta plaques in Alzheimer’s disease: phase 3 study. Alzheimers Dement, 2015. 11(8): p. 964-74.
- Sabri, O., et al., Beta-amyloid imaging with florbetaben. Clin Transl Imaging, 2015. 3(1): p. 13-26.
- Vos, S.J., et al., Prediction of Alzheimer disease in subjects with amnestic and nonamnestic MCI. Neurology, 2013. 80(12): p. 1124-32.
- Bloudek, L.M., et al., Review and meta-analysis of biomarkers and diagnostic imaging in Alzheimer’s disease. J Alzheimers Dis, 2011. 26(4): p. 627-45.
- Sperling, R.A., Karlawish, J., and Johnson K.A., Preclinical Alzheimer disease-the challenges ahead. Nat Rev Neurol, 2013. 9(1): p. 54-8.
- Jovalekic, A., et al., New protein deposition tracers in the pipeline. EJNMMI Radiopharmacy and Chemistry, 2017. 1(1).
- Roe, C.M., et al., Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology, 2013. 80(19): p. 1784-91.
- Dubois, B., et al., Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. The Lancet Neurology, 2014. 13(6): p. 614-629.
- Perret-Liaudet, A., et al., Risk of Alzheimer’s disease biological misdiagnosis linked to cerebrospinal collection tubes. J Alzheimers Dis, 2012. 31(1): p. 13-20.
- Kang, J.H., et al., Clinical utility and analytical challenges in measurement of cerebrospinal fluid amyloid-beta(1-42) and tau proteins as Alzheimer disease biomarkers. Clin Chem, 2013. 59(6): p. 903-16.
- Ng, S., et al., Visual Assessment Versus Quantitative Assessment of 11C-PIB PET and 18F-FDG PET for Detection of Alzheimer’s Disease. Journal of Nuclear Medicine, 2007. 48(4): p. 547-552.
- Perani, D., et al., A survey of FDG- and amyloid-PET imaging in dementia and GRADE analysis. Biomed Res Int, 2014. 2014: p. 785039.
- Piramal, NeuraCeq (florbetaben 18F) Summary of Product Characteristics. 2015, Piramal Imaging Ltd.
- EMA. Human Medicines: European public assessment reports. 2016 [cited 2016 July]; Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/landing/epar_search.jsp&mid=WC0b01ac058001d124.
- Johnson, K.A., et al., Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. Alzheimers Dement, 2013. 9(1): p. e-1-16.
- Johnson, K.A., et al., Update on appropriate use criteria for amyloid PET imaging: dementia experts, mild cognitive impairment, and education. J Nucl Med, 2013. 54(7): p. 1011-3.
- Scarsbrook, A. and Barrington S., Evidence-based indications for the use of PET-CT in the United Kingdom 2016, R.C.o.P. Royal College of Radiologists, Editor. 2016, RCR, RCP: London, UK.
- Bang, J., Spina, S., and Miller, B.L., Frontotemporal dementia. The Lancet, 2015. 386(10004): p. 1672-1682.
- Kobylecki, C., et al., A Positron Emission Tomography Study of [18f]-Florbetapir in Alzheimer’s Disease and Frontotemporal Dementia. Journal of Neurology, Neurosurgery & Psychiatry, 2013. 84(11): p. e2-e2.
- Barthel, H., Seibyl, J., and Sabri O., The role of positron emission tomography imaging in understanding Alzheimer’s disease. Expert Rev Neurother, 2015. 15(4): p. 395-406.
- Pontecorvo, M.J., et al., A randomized, controlled, multicenter, international study of the impact of florbetapir (<sup>18</sup>F) PET amyloid imaging on patient management and outcome. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 11(7): p. P334.
- NICE. Dementia – assessment, management and support for people living with dementia and their carers: GUIDANCE. NICE Guidance 2016 [cited 2016 June]; Available from: https://www.nice.org.uk/guidance/indevelopment/gid-cgwave0792.
About Vanessa Newman
Vanessa’s background is in neurology (epilepsy and Down’s syndrome) and more recently in the field of neuroimaging in dementia. She has worked at Piramal Imaging since early 2015 and during this time has had the pleasure of seeing how quickly this area of medicine is moving, with increasing methods and imaging diagnostics available for use with people living with dementia.
Date of preparation: July 2016. ©Piramal Imaging Ltd. UK/FBB/1015/0021
Piramal Imaging Ltd, Langstone Technology Park, Langstone Road, Havant, Hampshire PO9 1SA, United Kingdom
Piramal Imaging Ltd medical information enquiries: Medicalaffairs.imaging@piramal.com
Piramal Imaging Ltd media enquiries: inquiries.imaging@piramal.com
Piramal is a British Institute of Radiology corporate member.



 I did indeed meet Sir Godfrey on numerous occasions. His humility and “boffin style” of science greatly appealed. Some of the stories at the numerous events surrounding his memorial service were truly fascinating, including his inability to accept any machine which he could not understand without taking it to bits and then reassembling it!
I did indeed meet Sir Godfrey on numerous occasions. His humility and “boffin style” of science greatly appealed. Some of the stories at the numerous events surrounding his memorial service were truly fascinating, including his inability to accept any machine which he could not understand without taking it to bits and then reassembling it!




