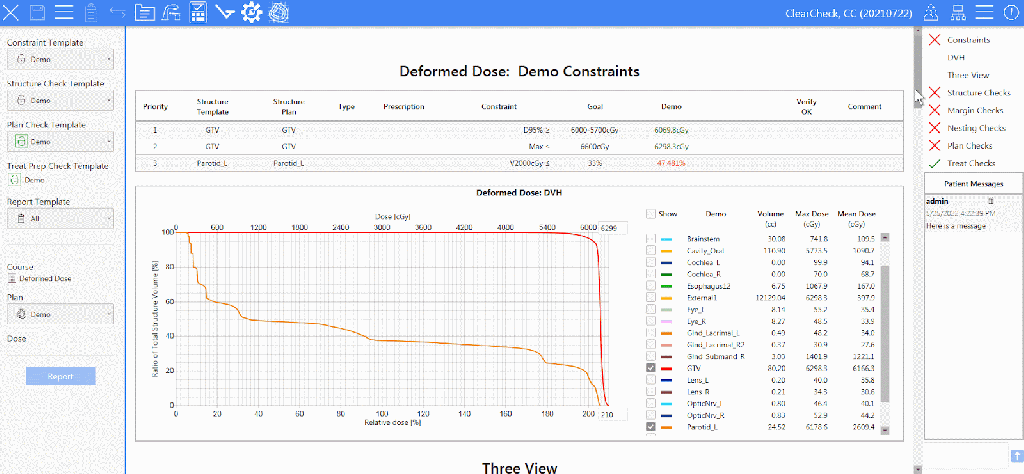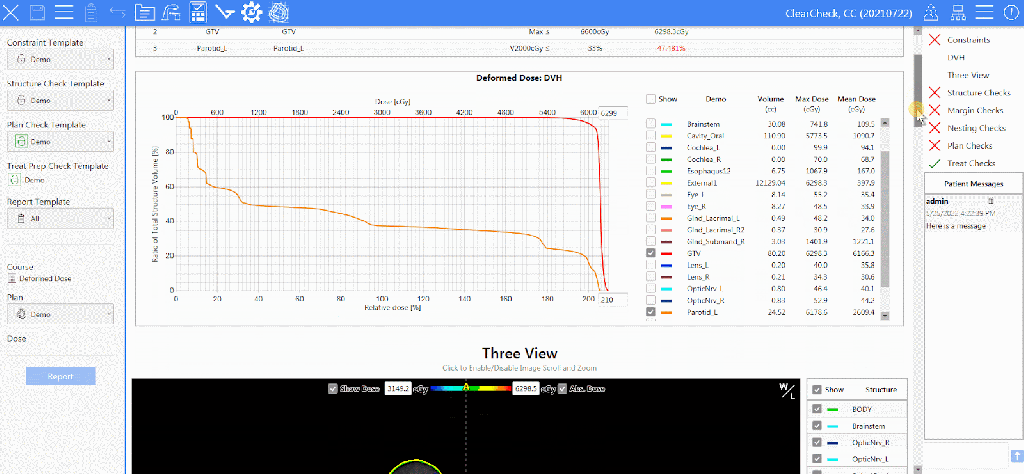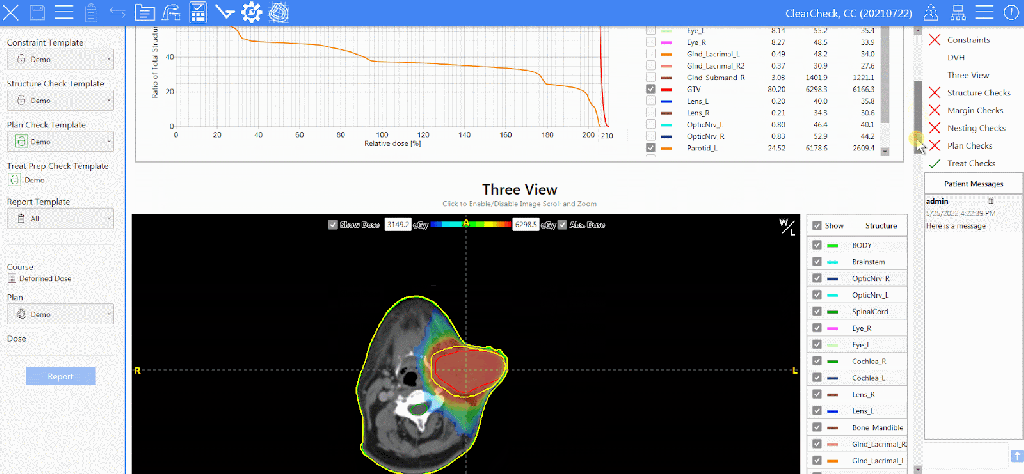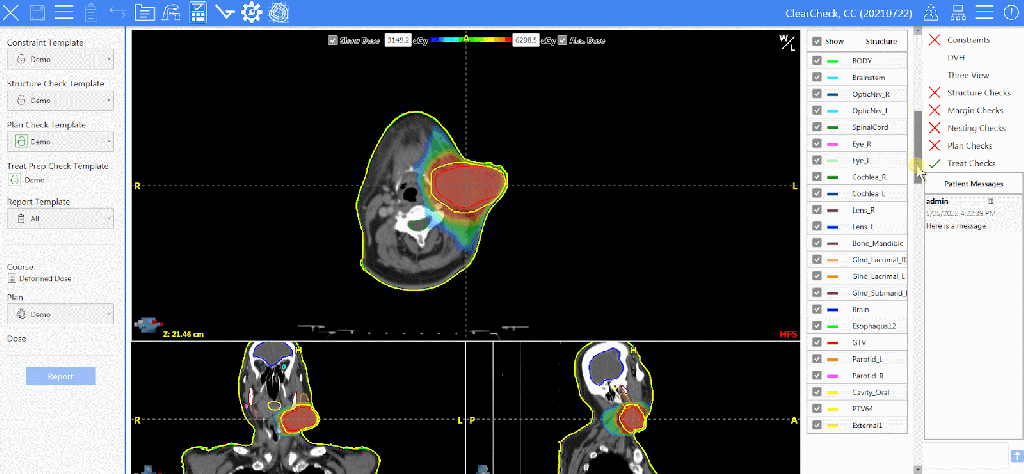
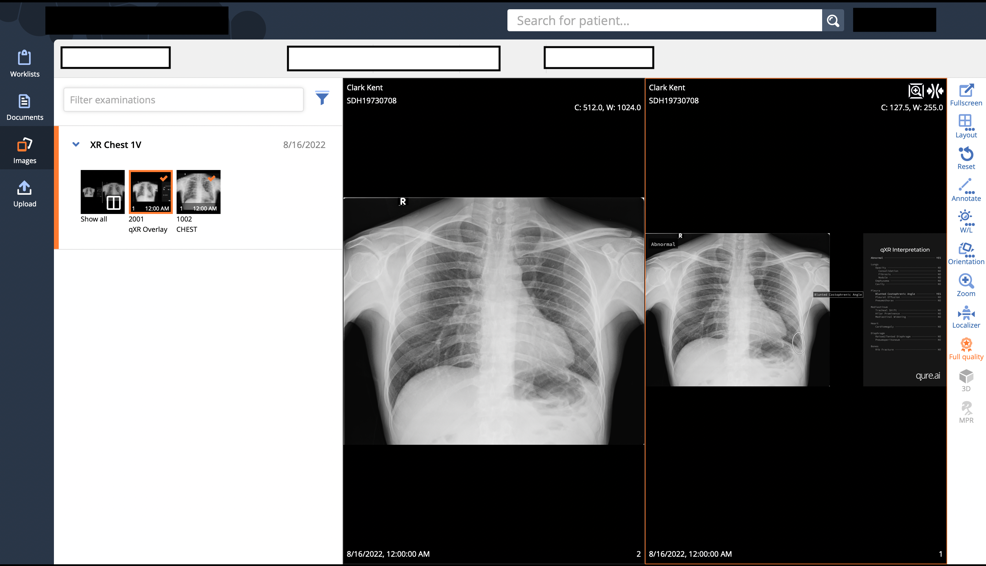
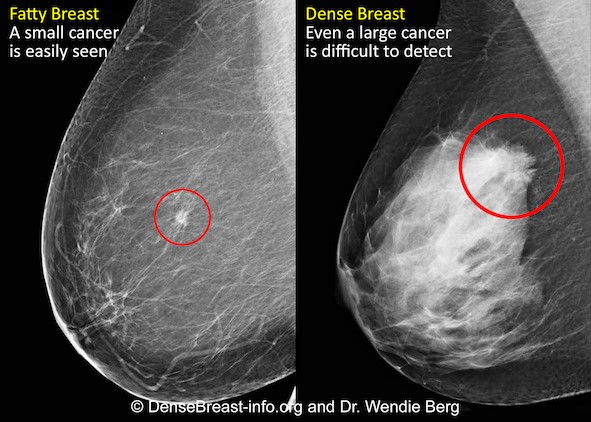
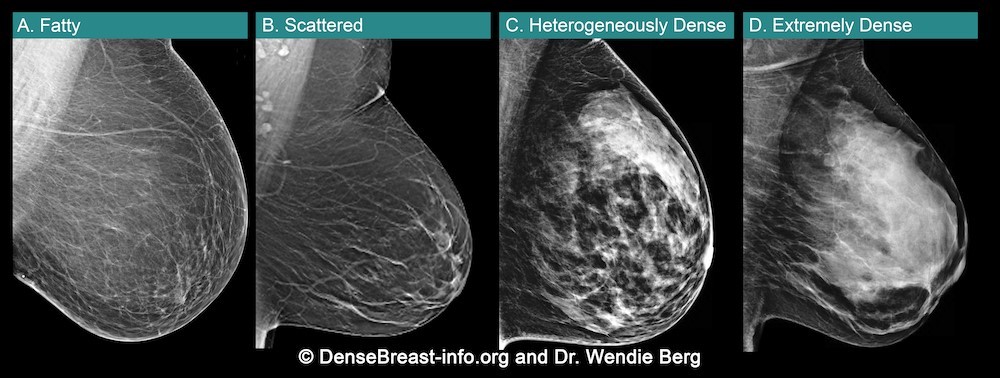
Kim Mason, an Audit and Research Radiographer for Mid Yorkshire Teaching Hospitals Trust, talks about their role as well as the value of radiographer engagement in research activities and how to get involved.
In December, 1895, Wilhelm Röntgen would x-ray the hand of Anna Bertha Ludwig, his wife, using a photographic plate. The new discovery lit a fire in the scientific community, and was so sensational that in the following year over 1,000 articles would be published on the topic of X-rays. Over the next 130 years, medical imaging has undergone many varied evolutions to become a cornerstone of modern-day medicine. All beginning with that first piece of research.
Radiography has exploded into a variety of modalities and specialisms from CT to Ultrasound to MRI; all driven by research and development. That is where I come in.
Hi, I’m Kim and I am an alternative-styled, funky-haired, septum-pierced, disabled Audit and Research Radiographer. That is to say, I’m fairly easy to pick out of a line up. I’m also passionate about education, research, and (of course) radiography. This, I’ve been told, is also very easy to pick up on.
I graduated with my radiography degree in 2018 and since then I’ve worked in a wide variety of departments, from plain film to nuclear medicine. Since I found something to love in every modality it never really mattered which one I was in. Prior to my current position, I’ve spent time as a Radiation Protection Supervisor, and well as a trainer for graduate and post graduate radiographers. Now I’m in Audit and Research, which is far different to anything I’ve done before.
So, what is an Audit and Research Radiographer?
My position is entirely based in research so I do not currently undertake any non-research imaging. However, I’m not entirely non-clinical. I get trained to undertake research scans on new equipment as required for me to carry out the necessary imaging for research trials. I also provide support to all trials requiring access to our radiography department, regardless of the imaging modality.
My job requires that I read a lot of trial protocols so that I can determine whether we have the necessary resources to undertake the trial. It can be hard work, and for me to be effective I have to have a good understanding of how each of our trusts’ imaging modalities function. I also need to have strong communication skills, especially in regards to explaining radiography perspectives to multi-disciplinary team members who may have little to no understanding of imaging. More than this, I need to be able to form and maintain good relations with all sides to ensure that effective communication can take place.
The work I do allows for better outcomes between radiology and research departments. It also affords me the opportunity to be a part of progress, seeking out better practice or improved technology for the future of imaging. In addition, I am able to undertake my own research, working towards my goals of further academic qualifications.
My role is important. I feel that I and my work contributions are valued by both the radiology and research teams. My pathway for personal development is clear and I am able to see the benefits in the set-up and management of the trials my Trust undertakes.
What is Radiography research and why is it important?
There is often research aimed at improving and advancing the field of diagnostic imaging. Currently there are trials into new scanning techniques; new equipment with the potentials for dose reduction and/or improved image quality and patient experiences; and the use of AI in imaging and reporting.
The benefits of improvements in our field are numerous. Every radiographer wants to give patients the best experience they can, however this is often at odds with the nature of our job. Requiring patients to hold uncomfortable positions when they are in pain or worried; perhaps having to go through narrow tubes which are sometimes incredibly loud; needing injections which makes them feel weird or mean they are radioactive and have to keep distance from other people at a time when they could really do with support. With research, we can aim to improve these experiences; reduce scan times or radiation exposures, wider bore scanners or open scanners, even finding new imaging or testing which removes the need for the ionising radiation all together!
Patients are at the heart of the NHS, and diagnostic imaging is often an area in which a good patient experience can be harder to provide (that is not to say that we don’t try!). With research, we have the potential to make those improvements to service, to provide for our patients in the way which we want to and the way that they deserve.
How can I get involved?
Often, radiography research is overseen by radiologists, doctors and orthopaedic surgeons, but there is no reason why radiographers shouldn’t also get involved. As we are the ones who use the equipment on a daily basis, consent and care for the patients during imaging, and come into contact with the faults and issues. Our profession contains a wealth of knowledge which can be used to improve all aspects of radiography.
You don’t need any academic qualifications to get started with research activities. In fact, many radiographers image research patients without being privy to the research aims. In a busy department, such patients are treated the same as any other in most regards. If you do image a research patient, perhaps look into the trial itself. As well as being interesting additional information, it can be used as material for CPD in the form of a case study or reflective piece. You may also discover potential ways to improve the patient experience within your department and help to enact future change.
Look into what your hospital requires for research involvement. The Good Clinical Practice (GCP) qualification, which is usually necessary, can be found for free as an online e-learning module. The NIHR website provides a lot of helpful information for getting started. NIHR also provides help for those who are wanting to gain further academic qualifications, such as through grant applications for fellowship awards. These are highly competitive but allow better access for NHS employees to undertake Masters or Doctorate level qualifications. The NIHR also run conferences for those who are new to research but interested in how they can take part.

You could also look at taking part in your department’s audits. Audits are a great way to check in on the health of your department, what you are doing well, and what you can improve on. Audit skills can also overlap with those necessary for research work, as well as provide possible avenues for research within your department.
How do I get a research job?
The roles of radiographers in research are expanding. Some hospitals offer clinical research radiographer positions, which give additional responsibility to train for and undertake specialised research imaging, often alongside a multi-disciplinary team. Other trusts may offer training for those wanting to aid research trials.
For research-specific roles, take a look at NHS jobs. You will find posts for Research Radiographers or Research Clinical Practitioners/AHPs. When I applied for my Audit and Research radiographer post, I had no specific research skills however I was well versed in audits and had learned about the processes of research in my own time. Enthusiasm goes a long way when applying for research roles, we need radiographers who are driven and raring to get stuck in.
There is so much experience and knowledge that radiographers have to offer research, and there’s so much improvement and advancement to be received in turn. I strongly encourage any radiographers to give it a try. You never know, you may get hooked!
To submit your research to a BIR journal find out more here:
BJR https://www.editorialmanager.com/bjr/default2.aspx
BJR Case|reports https://www.editorialmanager.com/bjrcr/default2.aspx
BJR|Open https://www.editorialmanager.com/bjro/default2.aspx
About Kim Mason
Kim Mason is a HCPC registered diagnostic radiographer, graduating from the University of Leeds with a 1st Class BSc Honours in Diagnostic Radiography in 2018. They have experiences in education both inside and outside of radiography, and have a passion for improving the radiography services in the UK.
Kim has multiple chronic conditions, and as such, they are an ambulatory wheelchair user. This has given them keen insight into the experiences of patients within the radiography department, having undergone imaging in most modalities as a patient. They have a vested interest in educating the public about radiography, and educating radiographers on improvements to patient care.