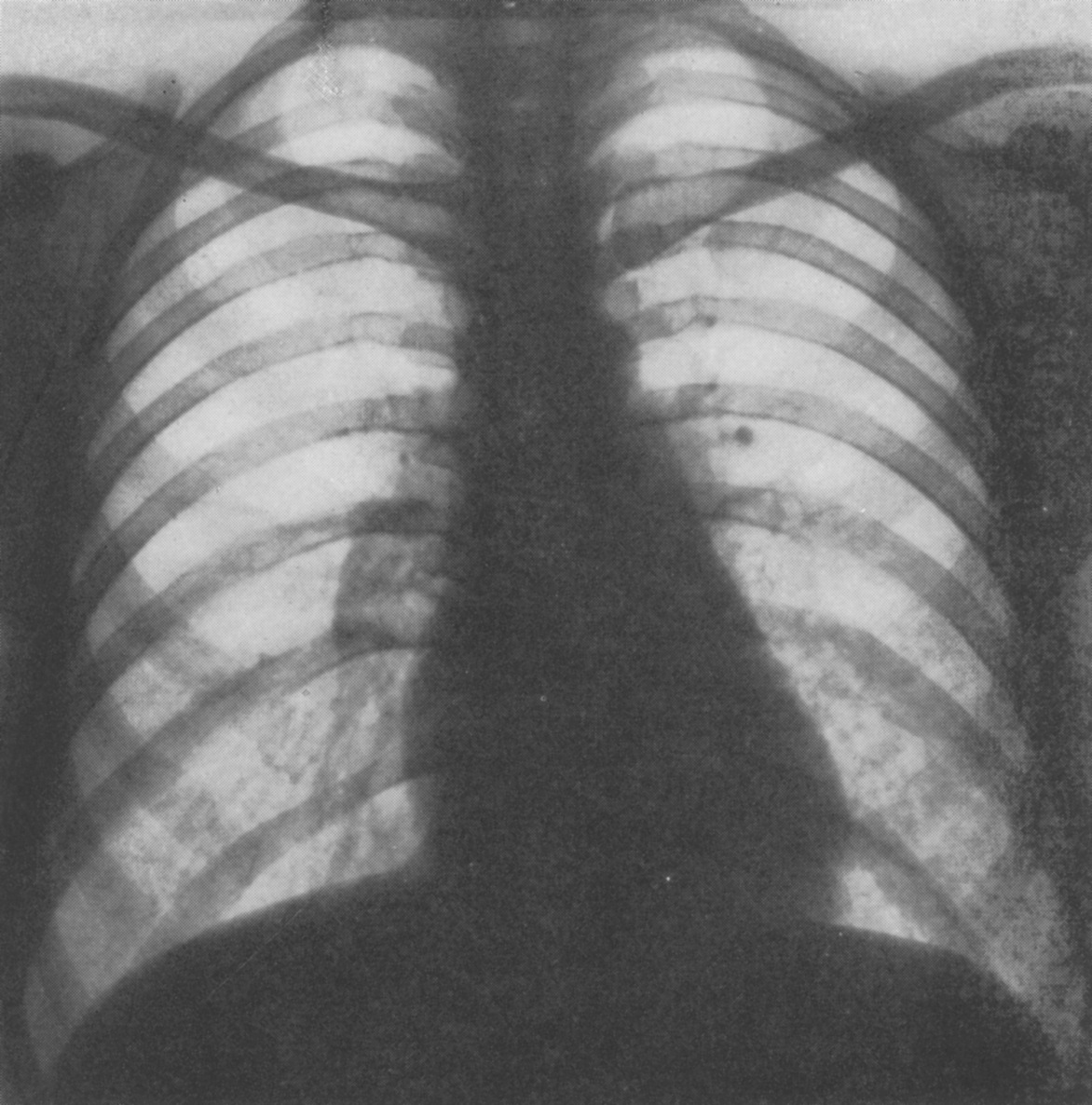
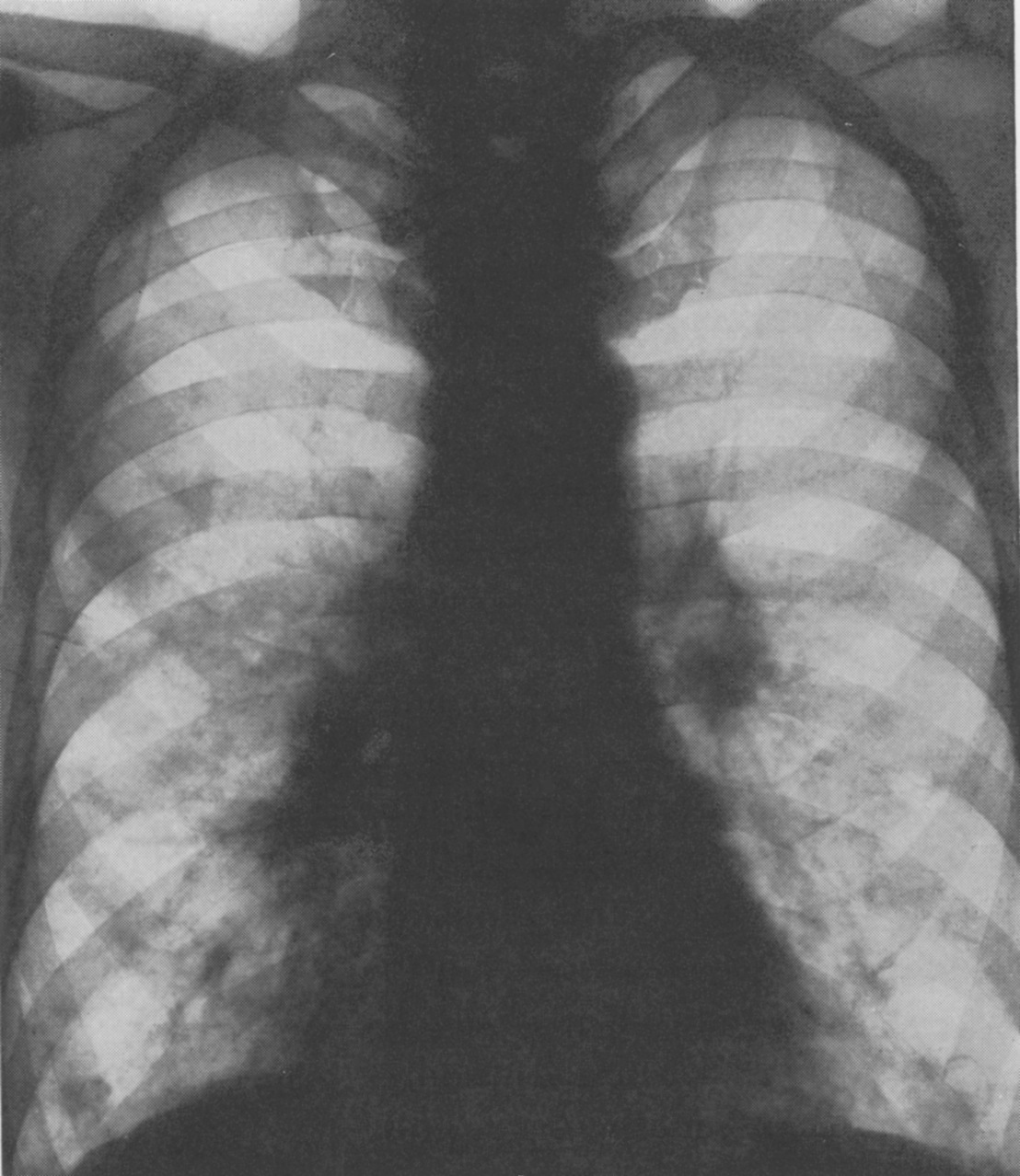
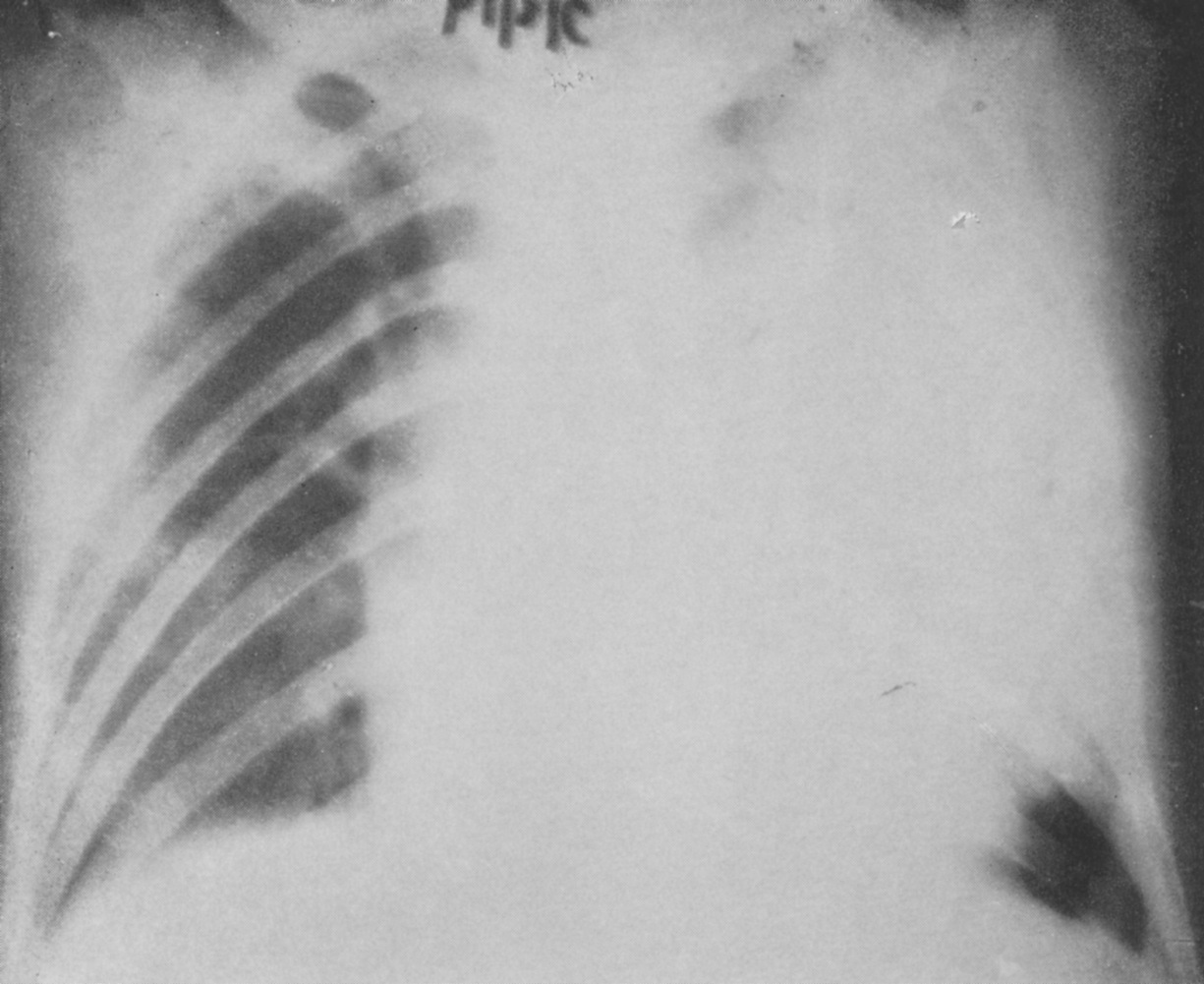One of the first doctors to use radiology to diagnose diseases of the nervous system, Artur Schüller began his first systematic survey of the skull just a few years after X-rays were first discovered. Here, Andrew Schuller, a distant cousin, describes his extraordinary academic and personal journey which led to his recognition as the “Father of Neuroradiology”.
Artur Schüller (hereafter Arthur Schuller) was born in the Moravian city of Brunn (now Brno in the Czech Republic) in 1874. Most of the Schullers in Brunn were involved in the textile industry but Arthur’s father, Jonas, was an ENT specialist. Arthur did well at the German-language secondary school and went on to enrol in the medical school of the University of Vienna, which at that time had an excellent international reputation. He graduated in 1899 sub auspiciis Imperatoris, a rare title only awarded by the Emperor to students who had scored perfect marks in all their school and university exams. This entitled him to select his post-graduate mentors and Arthur chose Wagner-Jauregg and Kraft-Ebbing, a combination that matched his interest in both anatomy and psychiatry. They sent him off to Berlin for 6 months in 1901 where he worked with Munk, Oppenheim and Krause who taught him about experimental physiology, clinical neurology and the diagnosis and treatment of brain tumours. By the time Schuller returned to work at the Allgemeine Krankenhaus, Vienna had already taken up Roentgen’s 1896 discovery of X-rays and Arthur was soon working with Guido Holzknecht, leader of Vienna’s radiological research efforts.
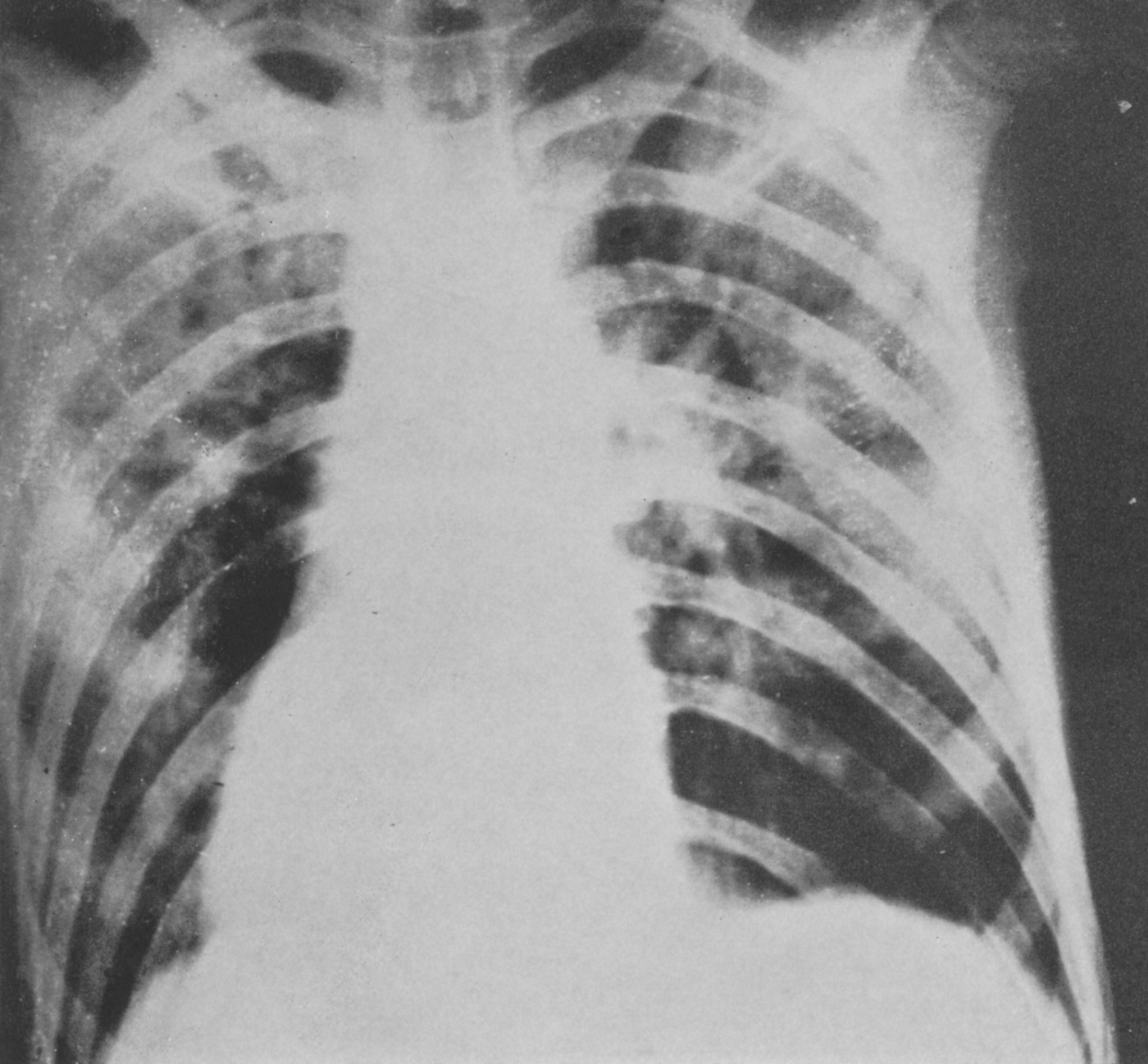
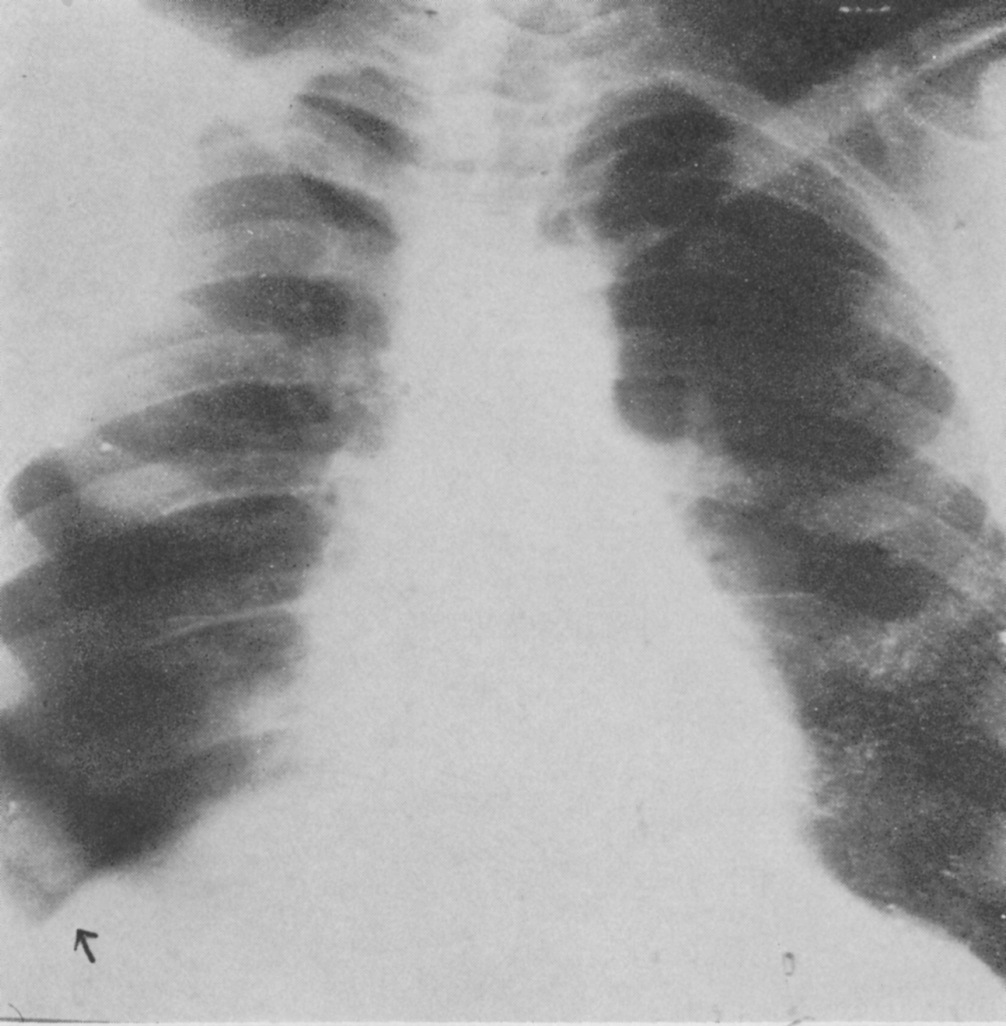
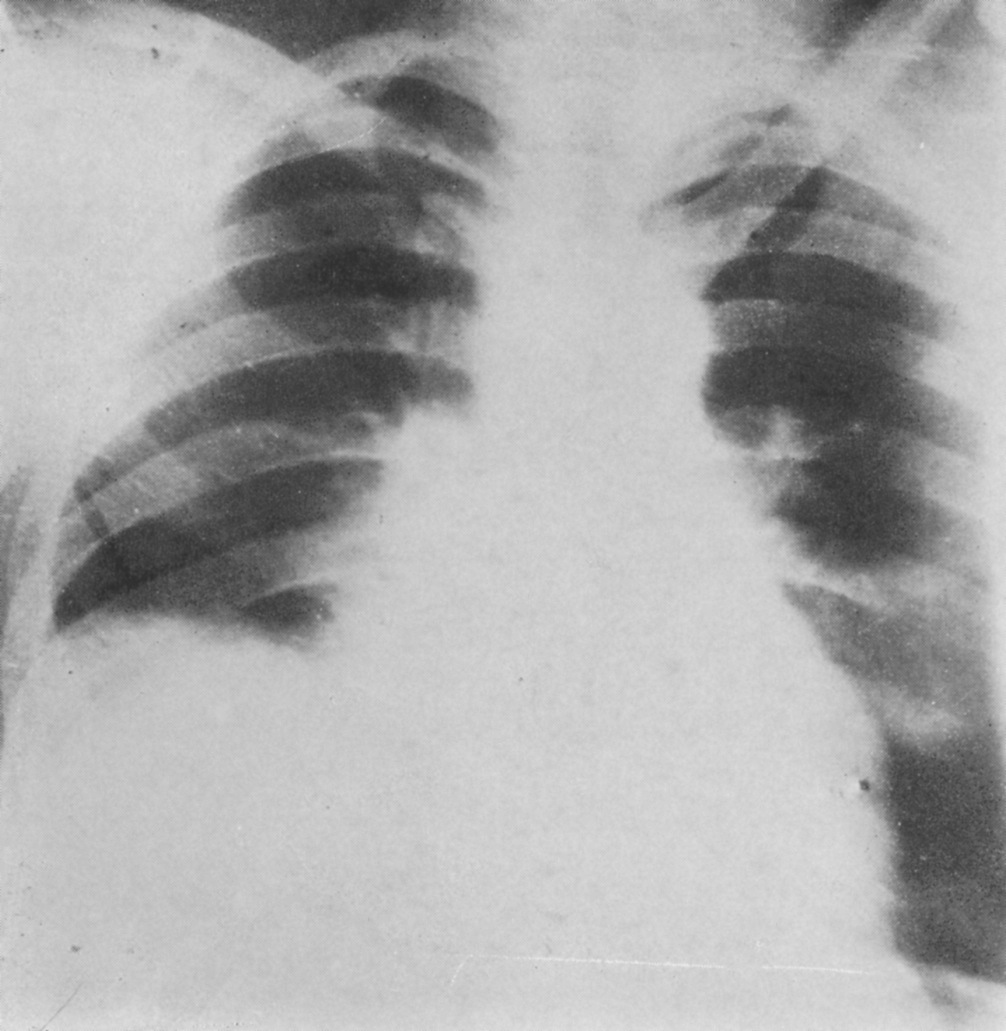
Schuller was the first to describe the role of X-ray in diagnosing diseases of the nervous system. Working with dried skulls from the museum and with live patients his painstaking analysis of countless X-rays enabled him to produce the first systematic survey of the radiology of the skull, which described both normal and pathologic anatomy. This book The Skull base on the Radiogram (Die Schädelbasis im Röntgenbilde: Archiv und Atlas der Normalen und Pathologische Anatomie) was published in 1905 and was followed in 1912 by Röntgen-Diagnostik der Erkrankungen des Kopfes (Röntgen Diagnosis of Diseases of the Head) which encapsulated his extensive work on a range of topics and was eventually translated and published in 1918 by C V Mosby in America under the auspices of the US Army (Incidentally, during WW2 Schuller, by that time in Australia, wrote about battlefield head injuries and worked in a military rehabilitation hospital).
Schuller’s interests ranged widely. From 1904 he was Director of the Children’s Hospital where he had worked in both the Neurology and the Psychiatric Clinics. But he maintained his experimental lab work and practice at the Allgemeine Krankenhaus. By 1907 he not only passed his Habilitation (PhD) but was also awarded Dozent status which allowed him to teach courses at the university as well as privately; he kept an X-ray machine in his home. When he was made a University Professor in 1914 he was the youngest in the medical faculty. In addition to his contributions to neurosurgical procedures (transsphenoidal approach to the pituitary, antero-cordotomy and hydrocephalic drainage) Schüller is associated with three bone diseases: the Hand Schüller Christian syndrome, osteoporosis circumscripta and cephalohaematoma deformans. But it is in his foundational work in forming the discipline of Neuroradiology that his outstanding contribution lies.
The financial stringencies and the political volatility that followed the collapse of the Austro-Hungarian Empire in 1918 had a serious impact on the ability of the Vienna Medical School to maintain its position at the forefront of medical research. But the continuing presence of so many prominent medical scientists enabled it to retain its international reputation. Along with Wagner-Jauregg Schuller was instrumental in expanding the existing post-graduate courses which attracted students from round the world. The US even established The American Medical Society of Vienna to administer the flow of almost 12,000 American students who enrolled in these courses between 1921 and 1938. Schuller continued to write papers and teach but he also consolidated his international reputation by travelling the world to lecture at conferences and clinics in the UK, Europe, Latin America and the US, where he lectured at the universities of Chicago, Johns Hopkins and New York and the Mayo clinic. In teaching and travelling he established strong personal contacts that would stand him in good stead including both Harvey Cushing and Walter Dandy in the US, both pioneering neurosurgeons with an interest in radiology. In 1935, while attending the Second International Neurological Congress in London, he met Hugh Cairns, an Australian neurosurgeon, who invited him to come to Oxford. Perhaps the apex of Schuller’s career was the central role he played in the first international congress of neuroradiologists. This, the First Symposium Neuroradiologicum was held in Antwerp in July 1939; the 22nd symposium will be held in 2022. There are some who assert that it was the Swede Lysholm who really established neuroradiology by developing contrast radiography, though it was Dandy who first wrote about ventriculography.
Schuller may not have pursued contrast radiography because of the lack of research facilities in interwar Vienna or because of the eclecticism and breadth of his intellectual interests but there is no doubt that his work was foundational for the routine radiography of the sella and its environs and the diagnosis of pituitary tumours. At the eighth Symposium Neuroradiologium in 1967 Bull and Fischgold declared “Without a shadow of doubt Arthur Schuller was the father of neuroradiology”. Schuller’s papers are still quoted in current literature and the Austrian Neuroradiological Society awards and annual Arthur Schüller Prize.

Schuller’s private life also flourished. In 1906 he had married Margarete Stiassni from a family of successful textile industrialists in Brunn. Arthur and Margarete were introduced at a post-opera supper party at the Sacher Hotel in Vienna where they shared their love of music. Arthur was a very competent violinist and played in the Vienna Medical School orchestra. In spite of the unsettled political situation, Vienna’s cultural life was still extraordinarily rich and the Schullers participated actively. They lived in a flat close to the university and the hospital and owned a house in Brunn and a weekend cottage by the Danube north of Vienna. They had a comfortable though not extravagant lifestyle. Indeed, the son of Arthur’s urologist cousin Hugo Schuller, who lived round the corner, reported that Arthur and Margarethe were somewhat parsimonious. Their two sons were born in 1908 and 1909. It may be that dedication to Arthur’s profession and his travel schedule had some impact on his relationship with Franz and Hans. It seems that they were closer to their mother’s family in Brunn than to the other Schullers in Vienna. They spent at least some of their teenage years living in Brunn with Margarete’s mother, to whom they were devoted, and both decided to join the family business rather than go to university, with fateful consequences.

The unstable political situation in Vienna deteriorated further in the late 1930s and after the Anschluss in March 1938 life for Jews became very difficult. Although Arthur and Margarete had been baptised as Roman Catholics in 1908 the National Socialist decrees categorized them as Jews. As such Arthur was only allowed to treat other Jews and in April 1938 he was officially “sent on holiday” from the university along with more than half of the members of the Medical Faculty, a purge that effectively set the Vienna Medical School back 60 years. The Nazi rampage through Vienna in November 1938 persuaded the Schullers that it was time to leave. Dandy had invited them to go to the US but Arthur was concerned about growing anti-semitism in academic medical circles there and decided on Australia. It seems that he was encouraged by two Australians who had attended his courses in Vienna and were now significant figures in Australian medical circles. Both John O’Sullivan and Sydney Sunderland were in Oxford in 1939 and to Oxford was where the Schullers fled when their Australian visas and their German Reich passports and exit papers arrived in early 1939. Arthur had followed up on Cairns’ invitation and in April was welcomed and attached to the labs of Le Gros Clark in Anatomy and Barclay at the Nuffield Institute for Medical Research. While there Oxford Arthur wrote a paper on the sub-arachnoid cisterns and their demonstration using a positive contrast agent which was published in BJR in 1940, 13.(148):pps 127-29
The Symposium in Antwerp finished on 29 July, 1939 and in the first week of August the Schullers left Croydon airport on a KLM flight which took over a week and 30 stops to reach Darwin and then Brisbane and eventually on to Melbourne. Their arrival was noted in the press in the main Australian cities.
What the Australians had organised was a position at St Vincent’s Hospital in Melbourne run by the Sisters of Charity, which is where John O’Sullivan was based in the Radiology Department. Also at St Vincent’s was Frank Morgan the first specialist neurosurgeon in Australia. Schuller enjoyed spending time in both departments viewing and reporting on all head X-rays and attending Morgan’s ward rounds and operations. He was liked and respected by staff at all levels. Curiously, however, the Medical Board of Victoria did not recognise his University of Vienna qualifications and he was not formally permitted to practise in Victoria till 1946. He was lent rooms where he did see patients who were referred to him. He continued to write papers – his last was published in 1950 – and, although he declined to attend the second Symposium Neuroradiologicum in Rotterdam in 1949, he was elected Honorary President and the paper he submitted was given pride of place.
In spite of his age Schuller’s attendance at St Vincent’s was constant, falling off only in his eighties. It was also stoical since on top of beginning to suffer from Parkinsons he had to bear the personal sorrow of family tragedy. Although they could have escaped, the Schullers’ two sons decided to stay in Brunn partly to care for their grandmother and partly to try to rescue the Stiassni family business. Both of them along with their grandmother and Hans’ wife and young daughter perished in Auschwitz in early 1943, though this news did not reach Australia till 1945. Arthur became increasingly depressed and even asked Morgan to perform a frontal lobotomy which Morgan refused to do. Arthur died in 1957 aged 83.

Margarete survived till 1971. She had taken to offering her services as a domestic help and, as such, worked for a number of Melbourne families. She cooked, ironed and looked after children. It is not clear why she did this. Since she left a substantial estate and her brother had continued to send monthly remittances from the USA financial need was probably not her main motivation. More likely she needed social company. She certainly embedded herself in some of the families for whom she worked. She was a devoted member of her local Catholic Church community.
Members of two of those families are featured in a 30 minute documentary film about the Schullers which is available for free viewing on YouTube at https://youtu.be/YhRLobn-Ubw
Also featured is Dr Keith Henderson who, as a young neurosurgeon at St Vincents, worked with and befriended Arthur. Henderson wrote a biographical memoir of the Schullers entitled Arthur Schuller Founder of Neuroradiology: a Life on Two Continents which Hybrid Publishers in Melbourne have just, in February 2021, published posthumously *. Henderson’s book contains substantially more detail about Schuller’s contributions to medical science than the film and it lists about half of the 300 papers he published.
*To order Arthur Schuller Founder of Neuroradiology: a Life on Two Continents in the UK https://www.amazon.co.uk/s?k=Arthur+Schuller%3A+Founder+of+Neuroradiology&i=stripbooks&ref=nb_sb_noss
To order Arthur Schuller Founder of Neuroradiology: a Life on Two Continents in Europe https://www.amazon.com/s?k=Arthur+Schuller%3A+Founder+of+Neuroradiology%3A+A+life+on+two+continents&i=stripbooks-intl-ship&ref=nb_sb_noss
About Andrew Schuller
Andrew Schuller was born in and educated at Oxford. He worked for over 30 years for Oxford University Press in New York and Oxford. Now retired, he spends much of the year in Australia and continues to be engaged in some publishing projects as well as family history. Andrew’s grandfather was a first cousin of Arthur Schuller, though it is not known if they ever met. By a strange series of coincidences, Andrew became involved in helping Keith Henderson in the writing of his memoir. It was at the suggestion of Austrian historians who have been recording the career and fate of Jewish medical practitioners in Vienna that Andrew embarked on making the film. He regrets that he did not know about Arthur much earlier when it would have been possible to talk to more people who knew him in Austria, Oxford and Australia.






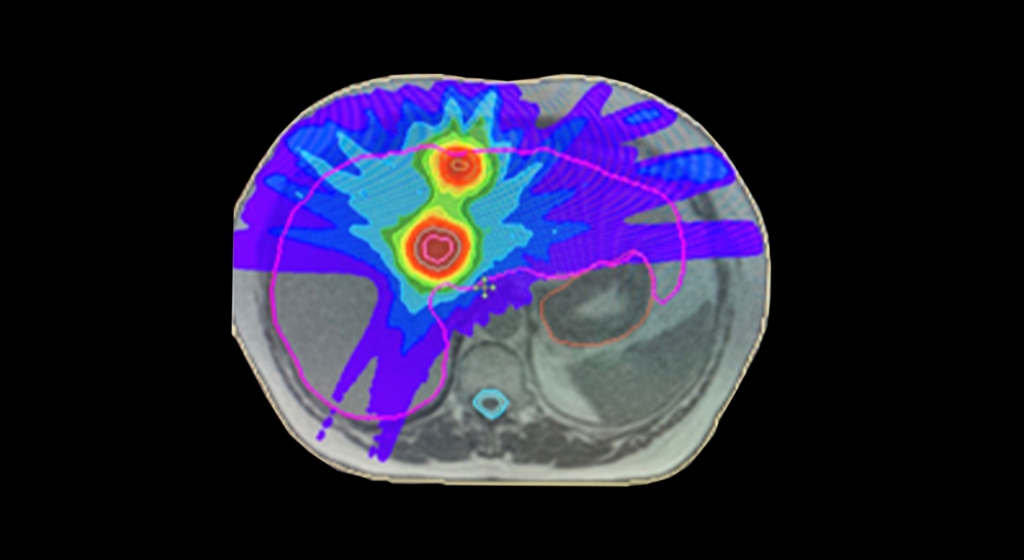
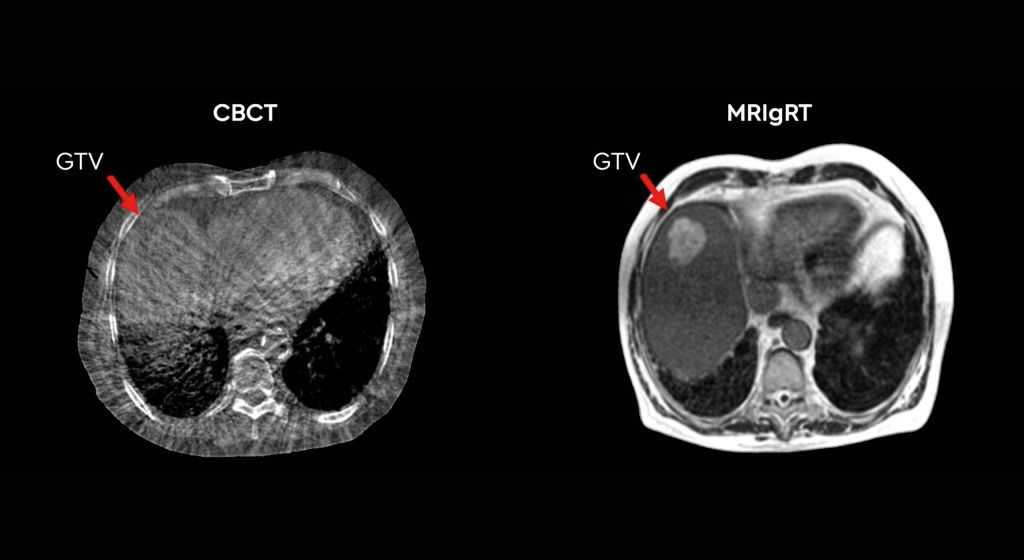







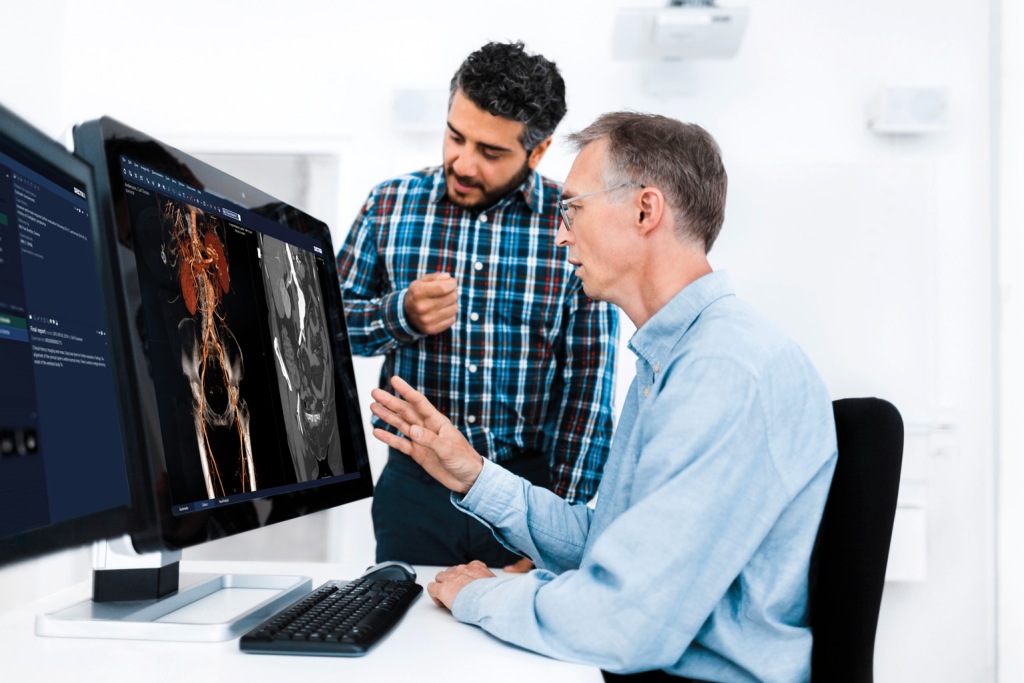
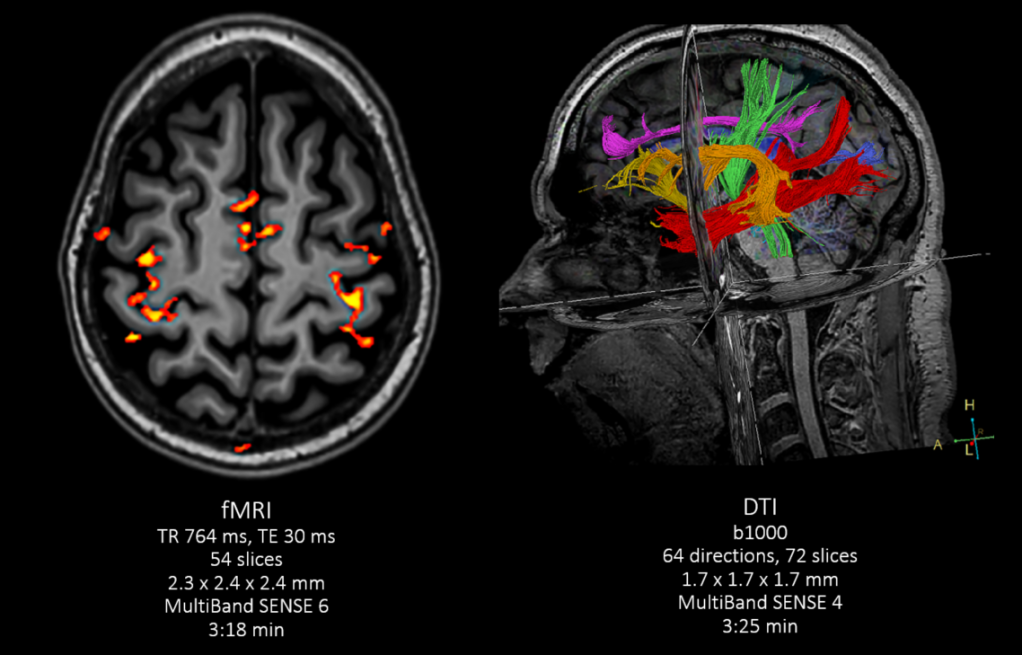




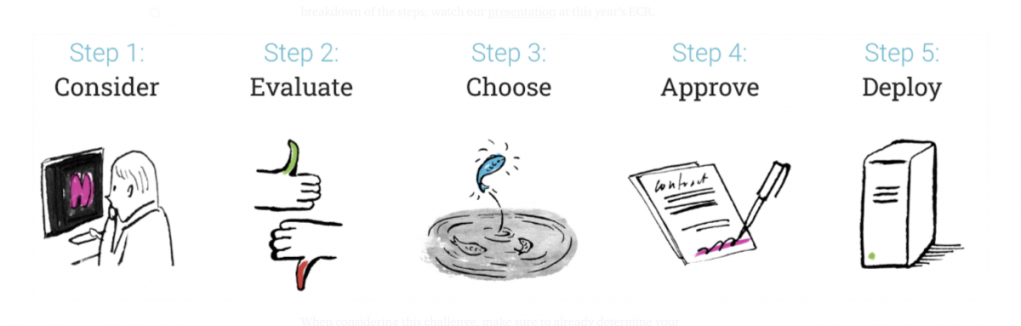


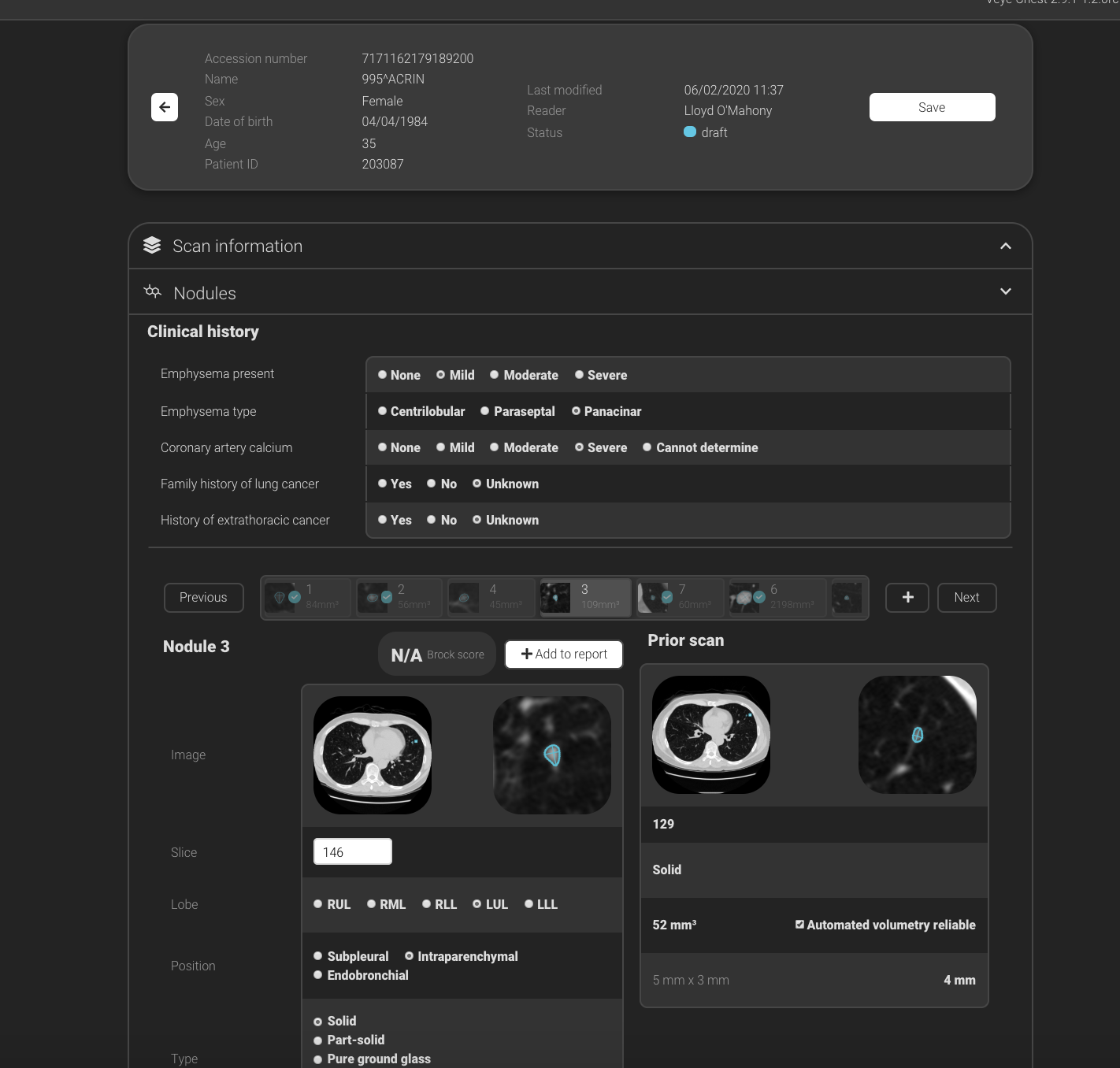

 Fodi Kyriakos explores how the COVID-19 pandemic could be the catalyst for change in radiology and encourages our community to grasp the opportunity to “seize the moment” and plan for recovery.
Fodi Kyriakos explores how the COVID-19 pandemic could be the catalyst for change in radiology and encourages our community to grasp the opportunity to “seize the moment” and plan for recovery.